Inhibit brain inflammation processes involved in disease pathogenesis
The relationship between protein pathology and neuroinflammation is complex with compelling evidence that chronic maladaptive neuroinflammation not only contributes to cellular toxicity produced by pathologic proteins but also impairs neuronal functions responsible for the clearance of these proteins (autophagy) further driving injurious neuroinflammation in a disease-progressing cycle. Beyond this fundamental role in propagating the neurodegenerative processes associated with pathologic proteins, there is now emerging evidence that activation of the innate immune system, such as from infectious pathogens, can precede, and then precipitate failures in autophagy that lead to the accumulation of the neurotoxic proteins that are the hallmark pathologies of these diseases. Thus, targeting disease-associated neuroinflammation may confer multiple benefits, including mitigation of the toxicity of misfolded proteins in an established disease state, and restoration of protein clearance mechanisms to slow the disease progression.
Reinforcing interplay between neurons and the immune system in neurodegenerative disorders
Reinforcing interplay between neurons and the immune system in neurodegenerative disorders
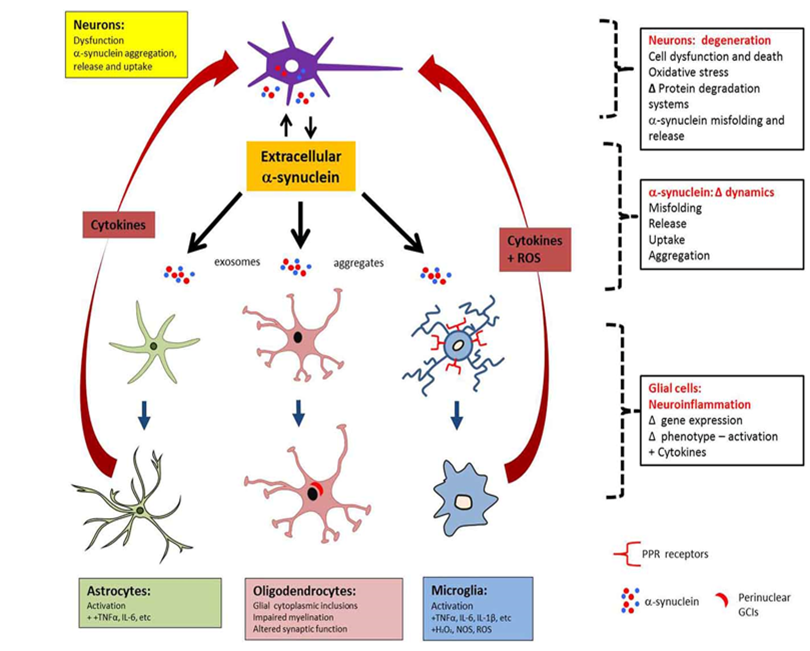
Adapted from Pountney et al., 2015
Neuropore Therapies has built an extensive array of cell based assays to elucidate the therapeutic potential of novel molecular targets and chemistries. These assays include those focused on inflammatory mechanisms in human monocytic cells (THP-1) and human microglia (the primary brain innate immune response cell) and those focused on protein clearance mechanisms (autophagy) in neuronal lines including Parkinson’s disease patient derived induced pluripotent stem (iPS) neurons (in collaboration with the University of Sydney). This integrated cellular system platform comprised of both immune and neuron-like cell lines provides Neuropore with unique insights into both the inflammatory and protein pathology aspects of neurodegenerative disorders and assessment of the therapeutic relevance of novel molecular targets and potential of new chemistries.
