rectify defects in the cellular mechanisms for clearing aggregates of misfolded proteins
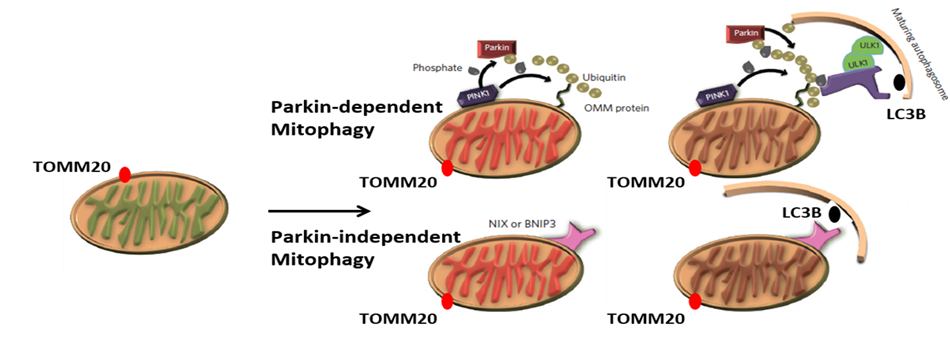
Numerous lines of evidence indicate that failure of protein quality control and clearance mechanisms is an underlying factor leading to the accumulation of misfolded and aggregated neurotoxic proteins in neurodegenerative disorders. Many of the genetic risk factors for Parkinson’s disease are associated with protein clearance mechanisms and further, the greatest risk factor for most neurodegenerative disorders is aging, which, itself, is associated with a decline in the efficiency of protein clearance mechanisms. Additional supporting evidence comes from animal- and cell-based studies where genetic or pharmacological impairment of specific protein clearance mechanisms recapitulates features of human neurodegenerative disorders. Taken together, these findings provide a strong rationale for a drug discovery strategy based on rectifying impaired protein clearance mechanisms. Neuropore Therapies is targeting three distinct lysosome-dependent protein quality control and clearance mechanisms for the treatment of neurodegenerative disorders. These are termed macroautophagy (MA), chaperone-mediated autophagy (CMA) and mitophagy (MIT).
Neuropore is targeting three distinct lysosome-dependent protein clearance mechanisms for the treatment of neurodegeneration: macroautophagy (MA), chaperone-mediated autophagy (CMA), and mitophagy (MIT)
Macroautophagy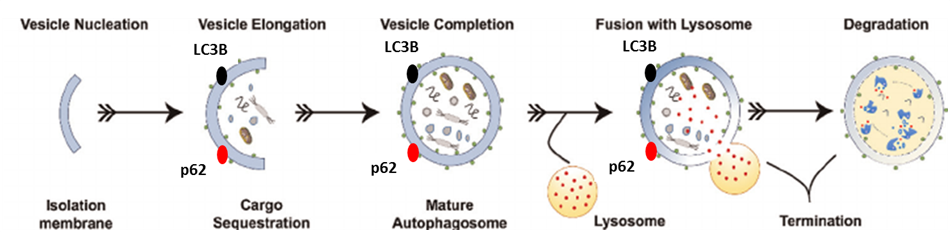
Chaperone Mediated Autophagy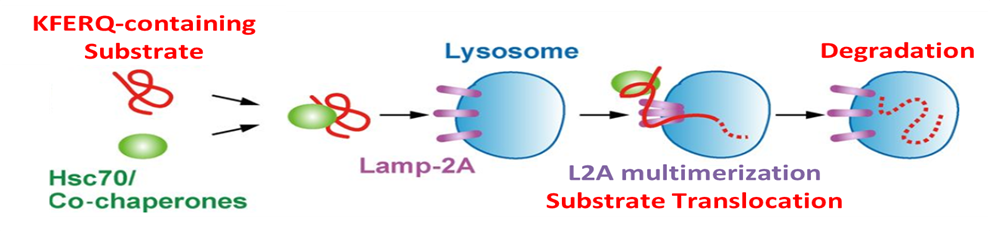
Mitophagy