Neuropore's Drug Discovery and Development Pipeline
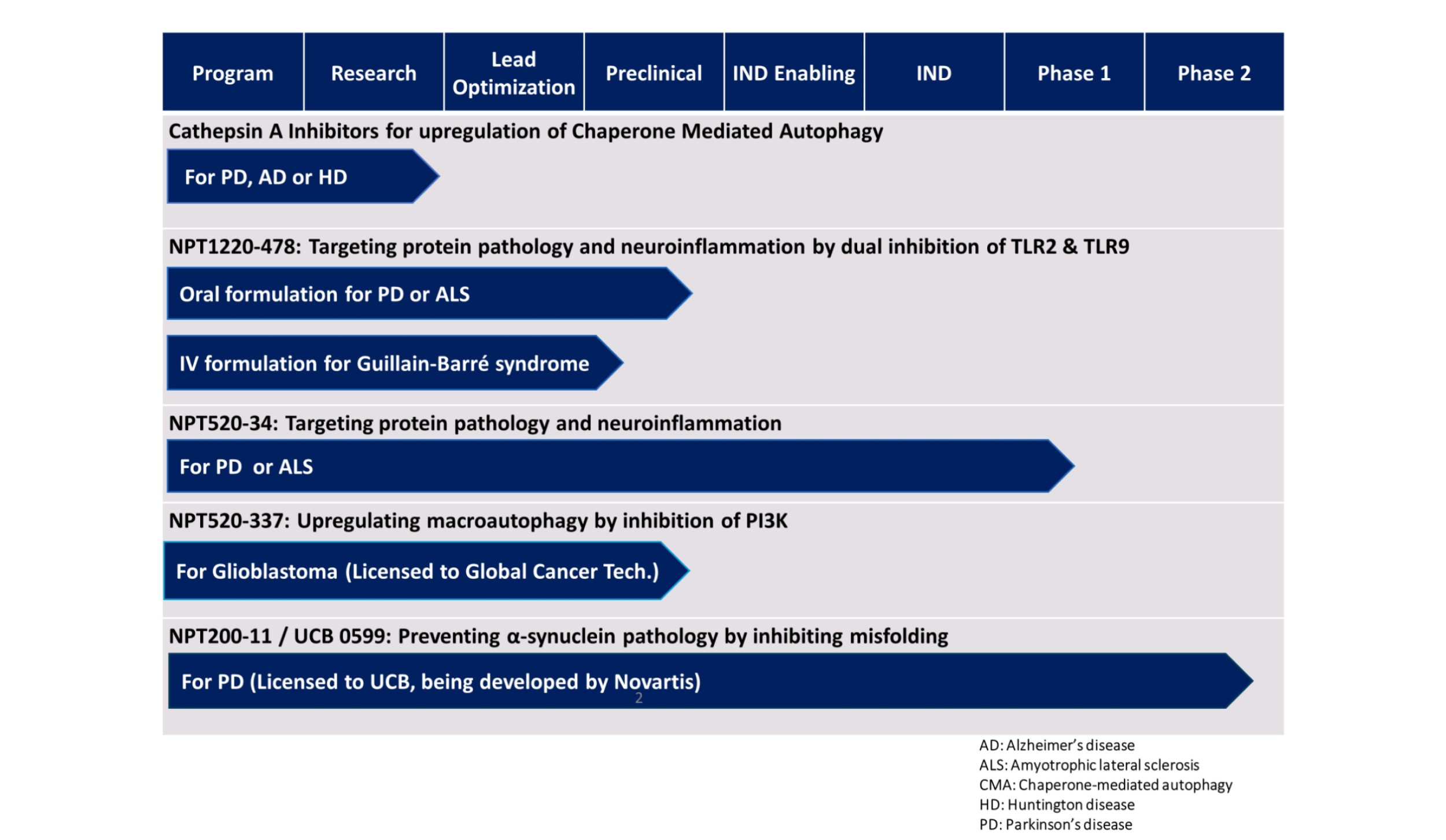
Following up upon the success of its first clinical candidate (UCB0599) under collaborative development with UCB Pharma for Parkinson’s disease Neuropore Therapies is currently advancing two exciting novel therapeutic candidates (NPT520-34 and NPT1220-478) as disease-modifying therapeutics for the treatment of neurodegenerative disorders.
NPT520-34 is clinical stage orally bioavailable small molecule that reduces astrocytic and microglial markers of neuroinflammation with robust beneficial effects on neuropathology and motor function in animal models of Parkinson’s disease. NPT520-34 also reduces the expression of markers of neuroinflammation and neuropathology in animal models of amyotrophic lateral sclerosis (ALS) and Alzheimer’s disease. NPT520-34 completed Phase 1 clinical studies in healthy volunteers in 2019.
NPT1220-478 is a potent and selective Toll-like receptor 2 (TLR2) & Toll-like receptor 9 (TLR9) dual antagonist that reduces markers of inflammation and neurotoxic protein burden in multiple cell systems including Parkinson’s disease patient-derived iPS neurons. With promising preclinical safety and pharmacokinetic profiles and potent target engagement in vivo already demonstrated, it is anticipated that NPT1220-478 will enter clinical development in 2022. Initial safety studies in healthy volunteers will include blood markers of target engagement, while subsequent studies in patients will incorporate brain imaging and perhaps peripheral biochemical markers of inflammation. Importantly, NPT1220-478 is just the first of multiple structurally diverse and promising small molecule TLR2/TLR9 antagonists being developed for applications in a range of central neurodegenerative and peripheral inflammatory disorders.
In addition to its anti-aggregation and neuroinflammatory therapeutic franchises, Neuropore Therapies has developed an integrated autophagy platform comprised of high throughput screening assays of different forms of autophagy (macroautophagy, chaperone-mediated autophagy and mitophagy). The NPT Autophagy Platform also includes biochemical assays to conduct hit confirmation as well as animal models of target engagement and efficacy. Compounded with Neuropore’s agile medicinal chemistry capabilities, the NPT Autophagy Platform is fine-tuned to efficiently identify novel autophagy targets and chemistries for small molecule drug-discovery.